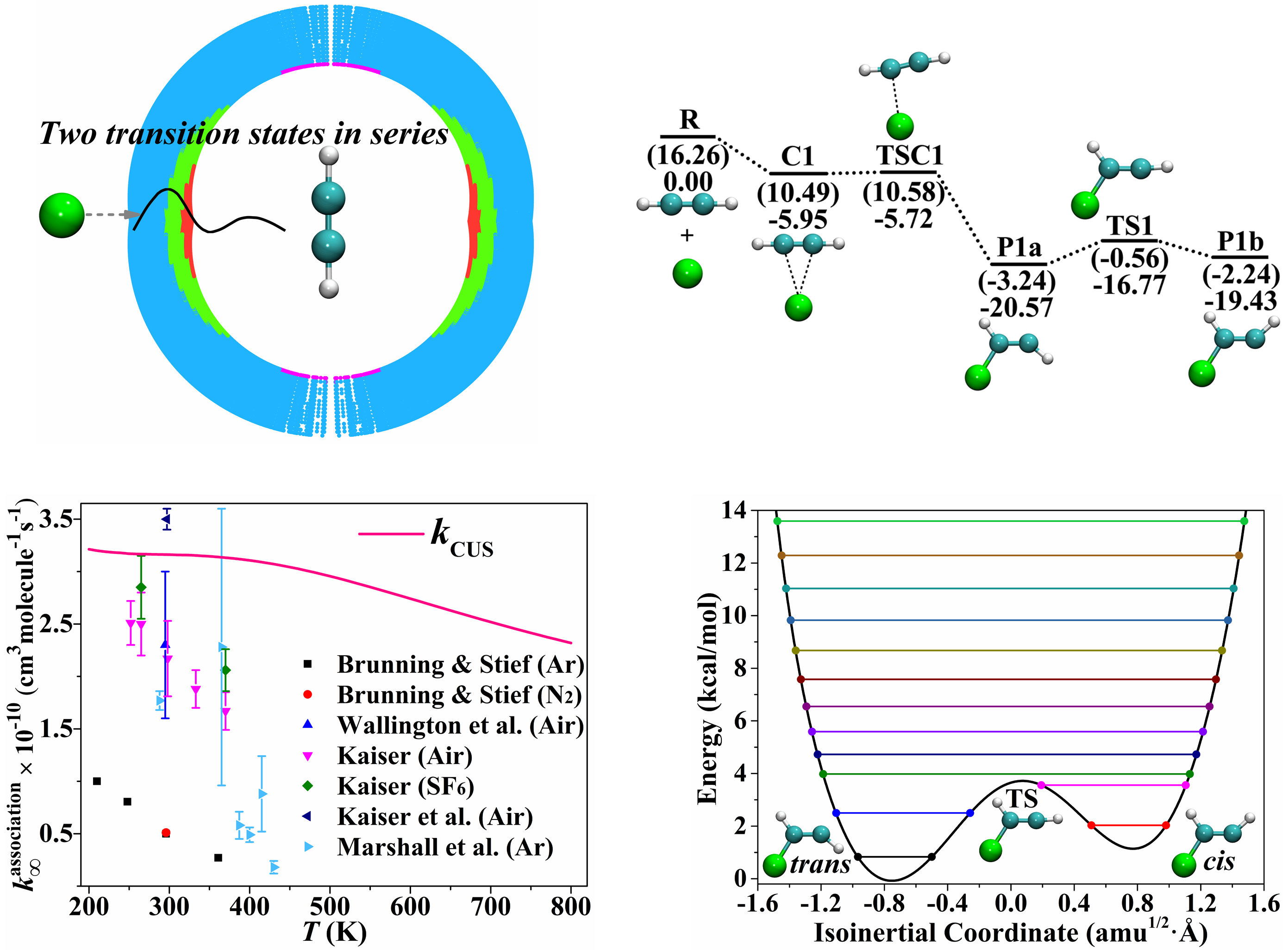
A key issue in chemical kinetics is advancing the theoretical framework to handle reactions beyond the domain of textbook transition state theory by including – for example – anharmonicity, barrierless transition states, transition states in series, and the effect of conformational flexibility on equilibrium constants. Other key issues are validating affordable electronic structure methods for direct dynamics and the direct calculation of high-pressure limiting rate constants, which are often obtainable experimentally only by extrapolation. We have addressed all these issues in a study of the prototype radical–molecule barrierless association reaction Cl + C2H2, and this work demonstrates the ability of recent advances in theoretical methods, when combined, to provide rate constants even for a difficult class of reactions in cases where experimental data are uncertain or missing.
“Association of Cl with C2H2 by Unified Variable-Reaction-Coordinate and Reaction-Path Variational Transition State Theory,” L. Zhang, D. G. Truhlar, and S. Sun, Proceedings of the National Academy of Sciences U.S.A. 117, 5610-5616 (2020). doi.org/10.1073/pnas.1920018117